
Genellikle, ilk 15 şirketin Ar-Ge bütçelerinin tamamında çok fazla dalgalanma görmüyorsunuzdur. Ar-Ge bütçelerinde biyoteknoloji ve bu yolda satınalmaların yeri tartışılmazdır.
You need a license to cut-and-paste this copyrighted news content.
Use this link to purchase your paid subscription ($200/year for individuals and $1,000/year for companies of every size): https://endpts.com/subscribe
Already a paid subscriber? Sign in to Endpoints News to remove this message.
The top 15 spenders in the global drug R&D business: 2017
You usually don’t see much annual fluctuation in the overall R&D budgets of the top 15 companies. The trend over the last few years has been to keep the lid on spending, particularly among the giants in Big Pharma. Companies didn’t cut much overall, but there was plenty of realignment going on as the industry refocused pipelines and continued a migration to the big hubs.
This past year, though, it was clear that a few companies wanted to turn up the heat in drug development, and this kind of fuel costs real money for companies that traditionally focus heavily on late-stage blockbuster drug research.
The top five in the business saw their collective spending jump by more than $5 billion, from 2015 to 2016, based on the annual numbers filed largely — though not entirely — with the SEC and gathered by Endpoints News. Two of those companies, Roche and the new number 2, a hard charging Merck, accounted for the lion’s share of the increase. (To be sure, some onetime non-R&D spending, such as Merck’s patent settlement with Bristol-Myers on Keytruda, figured in. But so did bread and butter spending.)
Gilead also saw a significant increase in research costs, with Eli Lilly — now off course following two bad setbacks for solanezumab and baricitinib — and the ever aggressive Celgene joining the action as they pressed the accelerator on new drug programs.
Curiously, the added spending coincided with a bad drop in new drug approvals in 2016. But they don’t correlate, and we’ve already seen that turnaround under way as regulators get busy with a brand new year — and soon a brand new FDA commissioner.
Paradoxically, one of the reasons why some of these R&D budgets have been rising is that development has been picking up speed. That’s abundantly clear now in the cancer field, where success with immuno-oncology has triggered a land rush mentality, with top players staking out as much territory as fast as possible. Anything left on the table won’t stay there for long, and first mover advantage can be critical. So invest now, reap your rewards later.
In the process, treating new oncology cases is gradually being revolutionized. And that’s not PR talk.
R&D reorganization, once aimed at massive cost cutting several years ago at places like Merck, GlaxoSmithKline, Pfizer and AstraZeneca, is still with us. But the form and substance has changed. Now these companies, along with a pressured Novartis and a bottom-line focused Amgen, have been looking to winkle out cost savings wherever they can be found. But many are also investing heavily in new R&D centers in San Francisco and Boston/Cambridge.
Meanwhile, some of the top players, like Sanofi, increasingly appear to be stuck. Their in-house organizations have been largely unproductive. Their partnerships are responsible for turning out the key new approvals.
We’ve seen some M&A deals, of course, but not an abundance of acquisitions from the big 10. Roche doesn’t feel it needs to. Merck hasn’t been buying much. AstraZeneca — which is increasingly cash restricted — has been selling off its disappointments, ginning some cash flow in the process.
The M&A standouts have been Pfizer (of course) and J&J, which carefully stepped up to buy Actelion’s portfolio for $30 billion. AbbVie is getting a rep for overspending on its deals.
Meanwhile Sanofi practically has to do a deal to prove it knows how after letting Medivation and Actelion slip through its fingers, and not just to higher bids. And Gilead is being ordered to get into gear by analysts who have been kept waiting too long.
We’re still waiting for the big one.
And that remains the key here. Perhaps after tax reform, if that happens, we’ll see some of those overseas billions put to use in buyouts. But it’s a long time coming.
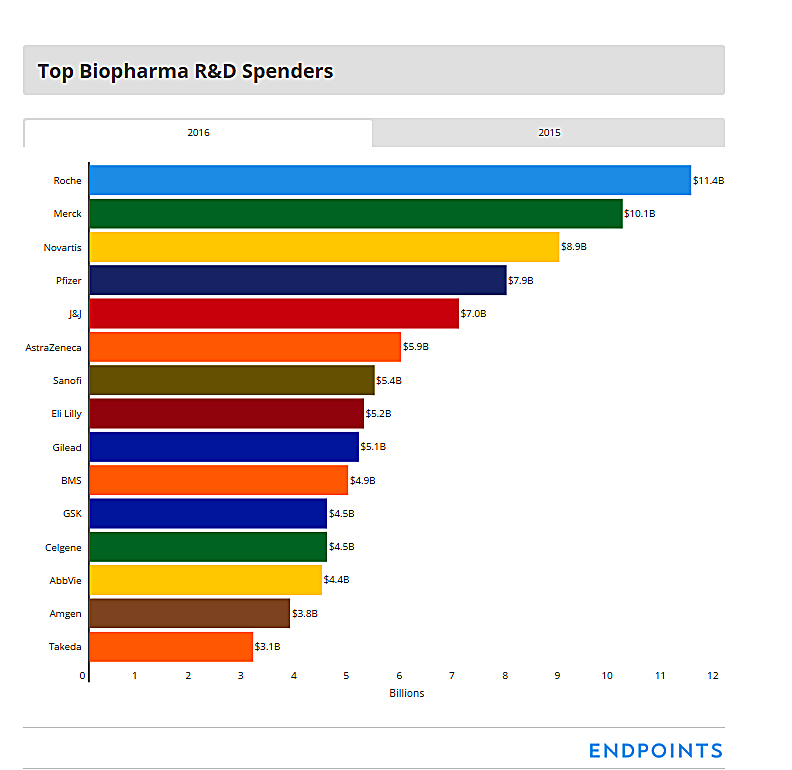
1. Roche
2016: $11.41 billion (11.53 billion CHF total: 10.15 billion CHF for pharma, the rest diagnostics)
2015: $9.2 billion (9.3 billion CHF total: 8.1 billion CHF for pharma, the rest for diagnostics)
Revenue: 50.5 billion CHF
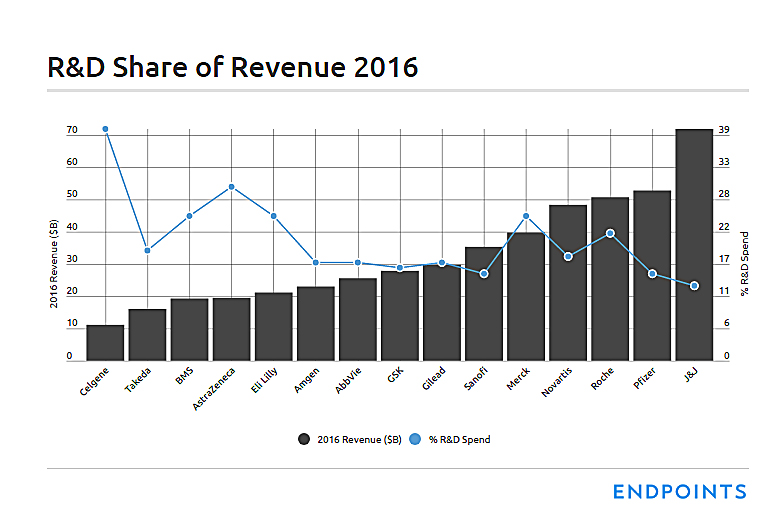
R&D as a percentage of revenue: 22%
Market: {$RHHBY}
R&D chief: Michael D. Varney, gRED; John Reed, pRED
A blockbuster budget delivers in Phase III
Give Roche credit where it’s due. The buttoned-down Swiss giant acquired Genentech and didn’t manage to kill the creative chemistry, a rare event in this business. And as a result, Roche has maintained a reputation for in-house innovation that has allowed its executive crew to remain largely exempt from pressure to join the expensive M&A game — at least for now.
Roche is a consistent player at the top of the list of R&D big spenders, shelling out considerable cash for two groups — Basel-based pRED and Genentech’s gRED in California — and still buys the occasional therapy for its pipeline. Just look at the recent deal it completed with Bristol-Myers Squibb, bagging the anti-myostatin Adnectin in development for Duchenne muscular dystrophy with $170 million upfront plus $205 million in milestones.
The big emphasis in the late-stage pipeline has been on blockbusters. And that has paid off with a recent approval for Ocrevus, a new multiple sclerosis drug looking to disrupt that market, following the big score last year on Tecentriq, the first of the PD-L1 cancer checkpoints.
Success for new cancer drugs opens the door to a multitude of combination studies, which Roche has engaged in eagerly when it comes to its big checkpoint contender. In addition, the giant scored another recent win for its cancer franchise, reporting that a key Phase III trial adding Perjeta to Herceptin and chemotherapy outperformed the two older standards alone in reducing the risk of death or relapse for early-stage breast cancer patients after surgery.
Analysts crowed that the clinical victory signals a major advance for Roche, likely adding billions to its megablockbuster oncology franchise.
Its new drugs are also beating a path forward in the clinic, as we saw when Alecensa beat out Xalkori in ALK-positive non-small cell lung cancer.
Roche has high hopes for emicizumab (ACE910) for hemophilia, but investigators continue to raise fresh questions about its safety following the death of one patient and a slate of serious adverse events in the pivotal Phase III. And it’s brought gantenerumab back from the dead for its Alzheimer’s Phase III pipeline, which also includes the newly added follow up late-stage study for crenezumab.




















